Alcohol Test A Level Chemistry . test for an alcohol: a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. understand the polarity and physical properties of alcohols; 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. Describe how ethanol is prepared; To determine between alcohols, add acidified potassium dichromate to the solution. Tollens’ reagent or fehling’s solution can then be used. use our revision notes to classify alcohols for a level chemistry. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. Then try our topic questions. They can be produced via two main methods. welcome to 2.10 alcohols.
from www.passmyexams.co.uk
Tollens’ reagent or fehling’s solution can then be used. understand the polarity and physical properties of alcohols; Then try our topic questions. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. They can be produced via two main methods. use our revision notes to classify alcohols for a level chemistry. test for an alcohol: 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. welcome to 2.10 alcohols. To determine between alcohols, add acidified potassium dichromate to the solution.
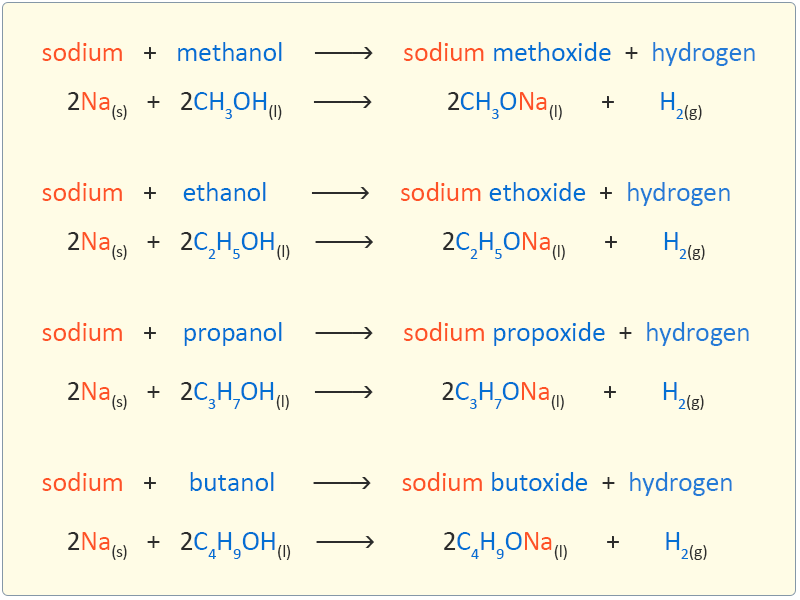
Reaction of Alcohols with Sodium Easy exam revision notes for GSCE
Alcohol Test A Level Chemistry 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. They can be produced via two main methods. 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. welcome to 2.10 alcohols. Then try our topic questions. test for an alcohol: To determine between alcohols, add acidified potassium dichromate to the solution. use our revision notes to classify alcohols for a level chemistry. Tollens’ reagent or fehling’s solution can then be used. Describe how ethanol is prepared; understand the polarity and physical properties of alcohols;
From www.pinterest.com
Classification of Alcohols Chemistry, Science chemistry, Chemistry notes Alcohol Test A Level Chemistry Tollens’ reagent or fehling’s solution can then be used. test for an alcohol: Describe how ethanol is prepared; They can be produced via two main methods. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. understand the polarity and physical properties of alcohols; 2.10 assessed homework task (mark scheme) for. Alcohol Test A Level Chemistry.
From mungfali.com
Lucas Test For Alcohols Alcohol Test A Level Chemistry welcome to 2.10 alcohols. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. Describe how ethanol is prepared; test for an alcohol: They can be produced via two main methods. To determine between alcohols, add acidified potassium dichromate to the solution. use our revision notes to classify alcohols for. Alcohol Test A Level Chemistry.
From www.organicchemistrytutor.com
Reactions of Alcohols — Organic Chemistry Tutor Alcohol Test A Level Chemistry Tollens’ reagent or fehling’s solution can then be used. Describe how ethanol is prepared; a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. welcome to 2.10 alcohols. To determine between alcohols, add acidified potassium dichromate to the solution. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that. Alcohol Test A Level Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Primary alcohol (1o alcohol Alcohol Test A Level Chemistry understand the polarity and physical properties of alcohols; test for an alcohol: 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. Describe how ethanol is prepared; They can be produced via two main methods. Tollens’ reagent or fehling’s solution can then be used. welcome to 2.10 alcohols. Then try our topic questions. To. Alcohol Test A Level Chemistry.
From www.pinterest.es
Oxidation of Alcohols Mechanisms and Practice Problems Chemistry Alcohol Test A Level Chemistry use our revision notes to classify alcohols for a level chemistry. 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. understand the polarity and physical properties of alcohols; They can be produced via two main methods. Tollens’ reagent or fehling’s solution can then be used. Describe how ethanol is prepared; test for an. Alcohol Test A Level Chemistry.
From www.linstitute.net
AQA A Level Chemistry复习笔记3.5.3 Oxidation of Alcohols翰林国际教育 Alcohol Test A Level Chemistry To determine between alcohols, add acidified potassium dichromate to the solution. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. Tollens’ reagent or fehling’s solution can then be used. Describe how ethanol is prepared; oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. test. Alcohol Test A Level Chemistry.
From www.slideshare.net
12 Chemistry Alcohols Phenols Ethers Test 01 Alcohol Test A Level Chemistry test for an alcohol: To determine between alcohols, add acidified potassium dichromate to the solution. understand the polarity and physical properties of alcohols; welcome to 2.10 alcohols. They can be produced via two main methods. use our revision notes to classify alcohols for a level chemistry. a primary alcohol will form an aldehyde and a. Alcohol Test A Level Chemistry.
From www.passmyexams.co.uk
Reaction of Alcohols with Sodium Easy exam revision notes for GSCE Alcohol Test A Level Chemistry a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. test for an alcohol: oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. Tollens’ reagent or fehling’s solution can then be used. understand the polarity and physical properties of alcohols; They can be produced. Alcohol Test A Level Chemistry.
From experhap.blogspot.com
CBSE Class 12 Chemistry Notes Alcohols, Phenols and Ethers Experhap Alcohol Test A Level Chemistry a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. welcome to 2.10 alcohols. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. They can be produced via two main methods. Then. Alcohol Test A Level Chemistry.
From chem.libretexts.org
10.1 Nucleophilic Substitution Reactions of Alcohols Forming Alkyl Alcohol Test A Level Chemistry To determine between alcohols, add acidified potassium dichromate to the solution. Describe how ethanol is prepared; understand the polarity and physical properties of alcohols; oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. They can be produced via two main methods. a primary alcohol will form an aldehyde and a. Alcohol Test A Level Chemistry.
From www.myxxgirl.com
Acidity Of Alcohols Alcohols Phenols And Ethers Class Chemistry My Alcohol Test A Level Chemistry To determine between alcohols, add acidified potassium dichromate to the solution. use our revision notes to classify alcohols for a level chemistry. Describe how ethanol is prepared; Tollens’ reagent or fehling’s solution can then be used. Then try our topic questions. welcome to 2.10 alcohols. a primary alcohol will form an aldehyde and a secondary alcohol will. Alcohol Test A Level Chemistry.
From www.studypug.com
Alcohols in Chemistry Compounds, Properties, and Applications StudyPug Alcohol Test A Level Chemistry Then try our topic questions. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. To determine between alcohols, add acidified potassium dichromate to the solution. welcome to 2.10 alcohols. Tollens’ reagent or fehling’s solution can then be used. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that. Alcohol Test A Level Chemistry.
From www.pdfprof.com
chemical test to distinguish between alcohol and alkene Alcohol Test A Level Chemistry To determine between alcohols, add acidified potassium dichromate to the solution. Describe how ethanol is prepared; oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. use our revision notes to classify alcohols for a level chemistry. understand the polarity and physical properties of alcohols; Tollens’ reagent or fehling’s solution can. Alcohol Test A Level Chemistry.
From getrevising.co.uk
Alcohols and Haloalkanes Revision Cards in A Level and IB Chemistry Alcohol Test A Level Chemistry 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. Tollens’ reagent or fehling’s solution can then be used. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. test for an alcohol: use our revision notes to classify alcohols for a level chemistry. understand the polarity and. Alcohol Test A Level Chemistry.
From www.organicchemistrytutor.com
Reactions of Alcohols — Organic Chemistry Tutor Alcohol Test A Level Chemistry 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. They can be produced via two main methods. test for an alcohol: Tollens’ reagent or fehling’s solution can then be used. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. a primary alcohol will form an aldehyde. Alcohol Test A Level Chemistry.
From dxokcqgvi.blob.core.windows.net
Test For Alcohol Chemistry A Level at Denise Yeary blog Alcohol Test A Level Chemistry Describe how ethanol is prepared; use our revision notes to classify alcohols for a level chemistry. understand the polarity and physical properties of alcohols; a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. Tollens’ reagent or fehling’s solution can then be used. test for an alcohol: To determine between alcohols,. Alcohol Test A Level Chemistry.
From dxokcqgvi.blob.core.windows.net
Test For Alcohol Chemistry A Level at Denise Yeary blog Alcohol Test A Level Chemistry welcome to 2.10 alcohols. Then try our topic questions. They can be produced via two main methods. use our revision notes to classify alcohols for a level chemistry. a primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. To determine between alcohols, add acidified potassium dichromate to the solution. oxidation reactions. Alcohol Test A Level Chemistry.
From oneclass.com
OneClass Alcohols and Phenols C. Oxidation of Alcohols 1. Color After Alcohol Test A Level Chemistry 2.10 assessed homework task (mark scheme) for the 2.10 test go to 2.11. oxidation reactions of the alcohols potassium dichromate k2cr2o7 is an oxidising agent that causes alcohols to. use our revision notes to classify alcohols for a level chemistry. understand the polarity and physical properties of alcohols; welcome to 2.10 alcohols. Describe how ethanol is. Alcohol Test A Level Chemistry.